|
Biograph
Working Experiance
|
Research Interests
For several years i have carried out collaborative research with a group based at the University of Manchester ,United Kingdom developing methods for the synthesis of 1,5-disubstituted tetrazoles , triazolinium salt and anovel iminophsphoranes { Ph3P=CR'CR"=NHMe}+otf- , according to the following published papers,Now im focusing in studying the influenc of the steric effect on formation of the tetrazoles and triazinium salts in the same reactions
1-The Chemistry of Nitrilium Salts. Part 3. The Importance of Triazinium Salts in Houben-Hoesch Reactions Catalyzed by Trifluoromethanesulphonic Acid
Abstract
In the presence of trifluoromethanesulphonic (triflic) acid, isobutyronitrile reacts with anisole, 1,3-dimethoxybenzene, resorcinol, 1,3,5-trimethoxybenzene, and phloroglucinol at room temperature to give acylation products.A study of the reactions of acetonitrile and isobutyronitrile with triflic acid has shown that these modified Houben-Hoesch reactions occur by initial cyclotrimerization of the nitriles to 1,3,5-triazinium triflate salts followed by a slow reaction of the salts with the aromatic substrate.Support for this comes from the isolation of 2-(4-methoxyphenyl)-2,4,6-tri-isopropyl-1,2-dihydro-1,3,5-triazine from the reaction between anisole, triflic acid, and isobutyronitrile.Similar 1,2-dihydro-s-triazines and their salts have been prepared by reaction of trimethyl-s-triazinium triflate with 1,3-(MeO)2C6H4, and tri-isopropyl-s-triazinium triflate with 1,3-(RO)2C6H4 (R = H or Me) and 1,3,5-(MeO)3C6H3; these salts are easily hydrolyzed to the corresponding aromatic ketones.The salt (1,3,5-Me3C3N3H2)+2(*)3SCF3 also reacts with o-phenylenediamine to give 2-methylbenzimidazolinium triflate in 83% yield.Nitrilium salts (RC(*)NMe)+<*<3SCF3 (R = Me, Ph, or PhCH2) undergo rapid reactions with 1,3-(MeO)C6H4 to form acylation products after hydrolysis, and N,N-dimethylaniline similarly affords 4-Me2NC6H4COMe on reaction with (MeC(*)NMe)+(*)3SCF3 at room temperature
=================================================================
2-Controlled Synthesis of 5-Substituted 1-Methyl-1H-Tetrazoles and 3,5-Disubstituted 1,4-dimethyltriazolium Salts from N-Methylnitrilium Trifluoromethanesulfonate Salts.
Abstract
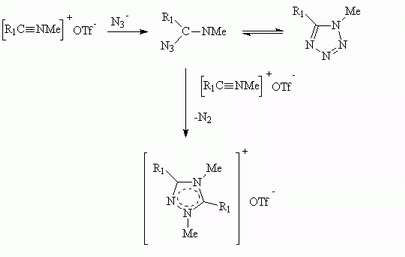
C-Alkyl and -benzyl N-methylnitrilium trifluoromethanesulfonate salts, (R1C=NMe)+ OTf- (R1=Me, Prn, Pri, But, PhCH2; OTf=O3SCF3), react rapidly under mild conditions with either sodium azide or tetramethylguanidinium azide in nitromethane to give the corresponding 5-substituted 1-methyl-1H-tetrazoles as the sole product in high yields.These reactions are independent of the mode of addition.C-aryl-N-methylnitrilium trifluoromethanesulfonate salts (R1=C6H6, 2-MeC6H4, 4-MeC6H4, 2,4-Me2C6H3) give mixtures of the corresponding tetrazoles and the triazolium salts with sodium azide.With tetramethylguanidinium azide the products depend upon the mode of addition.Addition of the nitrilium salt to the azide gives only the tetrazole, while inverse addition gives the triazolium salt in high yield.
=================================================================
3-Reactions of Stabilised Phosphorus Ylide with Nitrilium Trifluoromethanesulphonate Salts
Abstract
N-Methylnitrilium trifluoromethansulphonates react readily with methoxycarbonylmethylene(triphenyl)phosphorane to give methoxycarbonylmethyl(triphenyl)phosphonium trifluoromethanesulphonate and the new phosphonium trifluoromethanesulphonates (Ph3PC(CO2Me)=CRNHMe)+OTf- (R=Pri, Ph, 2-MeC6H4, 4-MeC6H4, Me) in moderate yields. 31P and 13C NMR spectroscopic data indicate significant contribution from the resonance form (Ph3PC(CO2Me)-CR=NHMe)+OTf- in the compounds
Last Updated sebtemper/ 2003