



Fire Science > The Fire
Figure 1-2 Fire
Fire is a form of combustion , is a chemical reaction involving two or more chemicals where the molecules will readily react with each other to form additional chemicals. It is typically orange, hot and smoky. Linguistically, the word fire refers to the combination of the brilliant glow and large amount of heat released during a rapid, self-sustaining burning of combustible fuel . Fire is not a state of matter: rather, it is an exothermic oxidation process by which heat and light energy are given out. Fire starts when a fuel with adequate supply of oxygen or other oxidizer is subjected to enough heat, and it is sustained by the further release of heat energy in the process, as well as a continuous supply of oxygen and combustible fuel. A match or lighter is usually used to start a fire, which can then propagate to other combustibles because matches and lighters are designed with materials of low burning point. Fire is extinguished when one or more elements of heat, oxidizer, or fuel is removed; this concept is used in the fire triangle . The unburnable solid remains of a fire are termed ash.
Flames can conduct electricity , as a small portion of any fire is ionized . This has been demonstrated in the laboratory and also in large wildfires that occur in the vicinity of power lines . This ability to conduct electricity is due to its partially plasmatic nature.
Fire Science > Fire Triangle

Figure 1-3 The fire triangle.
The Fire Triangle is a simple model, from the science of firefighting , for understanding the ingredients necessary for most fires . It has largely been replaced in the industry by the fire tetrahedron, which provides a more complete model, also described below.
The “triangle” illustrates the rule that in order to ignite and burn, a fire requires three elements — heat, fuel, and oxygen. The fire is prevented or extinguished by “removing” any one of them. A fire naturally occurs when the elements are combined in the right mixture (e.g., more heat needed for igniting some fuels, unless there is concentrated oxygen).
When a fire runs out of fuel it will stop. Fuel can be removed naturally, as where the fire has consumed all the burnable fuel, or manually, by mechanically or chemically removing the fuel from the fire. Fuel separation is an important factor in wild land fire suppression, and is the basis for most major tactics. Other fuels may also be chemically altered to prevent them from burning at ordinary temperatures, perhaps as part of a fire-prevention measure.
Without sufficient heat , a fire cannot begin, and it cannot continue. Heat can be removed by dousing some types of fire with water; the water turns to steam, taking the heat with it. Note that water will actually increase or spread some other types of fires. Separating burning fuels from each other can also be an effective way to reduce the heat. In forest fires, burning logs are separated and placed into safe areas where there is no other fuel. Scraping embers from a burning structure also removes the heat source. Turning off the electricity in an electrical fire removes the heat source, although other fuels may have caught fire and continue burning until the firefighter addresses them and their fire triangles too.
Oxygen may be removed from a fire by smothering it with aqueous foam or some inert gas (e.g., carbon dioxide , Halon ), dry chemicals, or enclose it where the fire will quickly use up all of the available oxygen. A candle snuffer uses this principle. Oxygen for the fire may also be instantaneously consumed, if only for a moment, by more ‘sophisticated' means such as using explosives to ‘snuff' an oil well gas fire. Once the gas fire is out, it is not hot enough to start again, but workers must be extremely careful not to create sparks.
Fire Science > Fire Tetrahedron
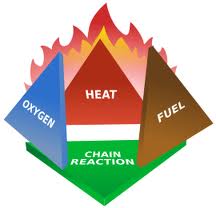
Figure 1-4 The fire tetrahedron.
Combustion is the chemical reaction that feeds a fire more heat and allows it to continue. With most types of fires, the old fire triangle model works well enough, but when the fire involves burning metals (known as a class-D fire in the American system of fire classifications, involving metals like lithium , magnesium , etc.), it becomes useful to consider the chemistry of combustion. Putting water on such a fire could result in the fire getting hotter (or even exploding ) because such metals can react with water in an exothermic reaction to produce flammable hydrogen gas. Therefore, other specialized chemicals must typically be used to break the chain reaction of metallic combustion and stop the fire.
Fire Science > Fire Square
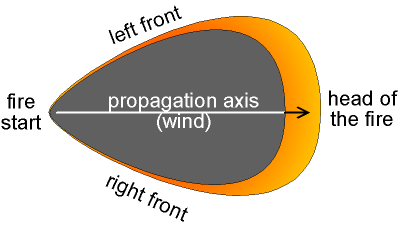
Figure 1-5 Propagation Model of Wild Fire
The Fire Square is a model created by fire ecologist Richard W. Halsey . It shows how catastrophic wild fires, like the 2003 Cedar Fire , are formed. It includes the three original elements from the Fire Triangle, but adds an extra side, showing Extreme Weather as another important element. Some examples of extreme weather would be El Niño , hot Santa Ana Winds , a long drought , or excessive vegetation growth. A wild fire can only be caused if one of these is present at the time. During the Cedar fire, Santa Ana winds were the cause for much of the fire's progress and re-kindling. The Fire Square was shown on an edition of The Weather Show.
JordanFire.Net









